Introduction: Intravenous (IV) iron is routinely used throughout various medical specialties in addition to hematology, including nephrology, cardiology, obstetrics, gastroenterology, and neurology. While all formulations are considered safe, those with iron dextran (ID) are reported to have a relatively higher incidence of anaphylaxis, often prompting a test-dose prior to full administration. Recently, there has been an ongoing national shortage of ID, leading to higher usage of alternate formulations and need for further characterization of usage and safety.
Methods: The Epic electronic health record was searched using the Slicer Dicer function to obtain information regarding intravenous iron administrations from 01/01/2021 to 08/31/2022 at the various Mayo Clinic sites throughout the United States. Adverse events were evaluated through the internal patient safety reporting system for adverse drug reactions. Serious adverse events (SAEs) were defined as those events requiring epinephrine administration, anaphylaxis, hypotension, tachycardia, or an allergic reaction (hives, rash, chest tightness, eye swelling) within 24 hours of IV iron infusion. Non-serious adverse events (NSAEs) were defined as those events requiring administration of an antihistamine, steroid, or reported gastrointestinal disturbance within 24 hours of IV iron infusion.
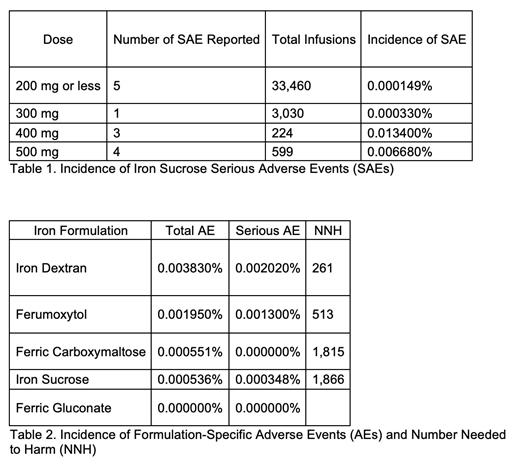
Results: A total of 49,310 infusions were administered during the analyzed period and included the use of five formulations: ID, iron sucrose (IS), ferumoxytol (FM), ferric carboxymaltose (FC), and ferric gluconate (FG). Infusions were distributed amongst Rochester (n=23,899; 16%ID, 77%IS, 5%FM, 2%FC, 0%FG), Florida (n=5,755; 6%ID, 43%IS, 34%FM, 9%FC, 8%FG), Arizona (n=5,123; 4%ID, 81%IS, 3%FM, 11%FC, 1%FG), and the Health System (n=14,533; 3%ID, 85%IS, 9%FM, 2%FC, 1%FG). A total of 49 AEs were reported (59% SAEs; 41% NSAEs), representing 0.099% of all infusions. Most AEs were from IS (n=20; 41%), with ID (19; 39%), FM (9; 18%), and FC (1; 2%) accounting for the remainder. Both SAEs (n=13) and NSAEs (n=7) were reported with IS, with most SAEs occurring on the first dose (85%) and classified as hypotension (39%), allergic (31%), anaphylaxis requiring epinephrine (15%), and tachycardia (15%); no pre-medications were given. These IS AEs were dose independent (Table 1). The incidence of AEs for all IV iron formulations was less than 0.01% (Table 2).
Conclusions: While ID was found to have a relatively higher incidence of AEs, all IV iron formulations were found to be safe. The most frequently used formulation was IS, which had a low incidence of AEs even at higher doses, suggesting feasibility for minimizing the number of infusions required to achieve therapeutic levels. Although all formulations demonstrated safety, each institution will likely individualize formulary preferences based upon location of administration (inpatient vs. outpatient), input from the various medical specialties, patient comorbidities, reimbursement patterns, and logistical constraints such as infusion chair time.
Disclosures
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal